Based on our experiment that we have done, we can determine protein concentrations by using Biuret, absorbance at 540nm and Lowry assay which the absorbance at 750nm. We use spectrophotometer to determine the absorbance of the protein. Below are the result for the reading of different gelatin concentration.
Results
Biuret

Lowry assay
Table 3 : The absorbance of light at 750nm for different gelatin concentration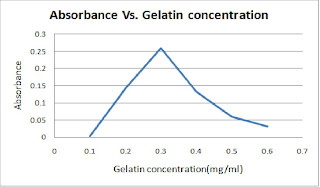
Based on the results, the suitable method of determining protein is by using Biuret assay. This is because the readings that we gain from the results is more accurate than using the Lowry assay. This method is the most linear because its color depends on a direct complex between the peptide bonds of the protein and Cu(2+) ion. It is not highly sensitive since the complex does not have a high extinction coefficient. The Lowry method relies on two different reactions.
This method is dependent on the presence of aromatic amino acids in the protein. First, a cupric/peptide bond complex is formed and then this is enhanced by a phosphomolybodate complex with the aromatic amino acids. Overall, about 10 to 50 times more sensitive than the Biuret method. The Folin Standard Curve is usually not perfectly linear, in the experiment, they are usually quite linear if the assay is properly done. Many substances interfere with the Folin assay for protein. The first reaction is the formation of a copper ion complex with amide bonds, forming reduced copper in alkaline solutions. This is called a Biuret chromophore and is commonly stabilized by the addition of tartrate .
The second reaction is reduction of the Folin-Ciocalteu reagent (phosphomolybdate and phosphotungstate), primarily by the reduced copper-amide bond complex as well as by tyrosine and tryptophan residues. The reduced Folin-Ciocalteu reagent is blue and thus detectable with a spectrophotometer in the range of 500 to 750 nm. The Biuret reaction itself is not very sensitive. Using he Folin-Ciocalteu reagent to detect reduced copper makes the Lowry assay nearly 100 times more sensitive than the Biuret reaction alone. Several useful modifications of the original Lowry assay have been developed to increase the dynamic range of the assay over a wider protein concentration ), to make the assay less sensitive to interference by detergents ( and to first precipitate the proteins to remove interfering contaminants.
The Lowry assay is relatively sensitive, but requires more time than other assays and is susceptible to many interfering compounds. The following substances are known to interfere with the Lowry assay: detergents, carbohydrates, glycerol, Tricine, EDTA, Tris, potassium compounds, sulfhydryl compounds, disulfide compounds, most phenols, uric acid, guanine, xanthine,magnesium, and calcium. Many of these interfering substances are commonly used in buffers for preparing proteins or in cell extracts. This is one of the major limitations of the assay.
Below are the results for the absorbance of different grades of the egg.
Table 2 : The absorbance of different grade of egg.
Grade does determine the protein content in eggs. The grade is determined by the interior quality of the egg and condition of the egg shells. After eggs are laid, gathered and washed, they get graded and sized before they're packed into cartons. The grade is decided by checking both the outside and the inside of the egg. On the outside, the checker looks to see if the shell is clean and unbroken and has a normal shape and texture - without bumps, ridges, thin spots or rough areas. The shell color doesn't matter.
On the inside, the checker looks to see if the white is firm, thick and clear. The checker also looks to see if the yolk is the right size and shape and has no blemishes. Through the shell, the checker can see the size of the air cell, too. The smaller the air cell, the higher the grade. Eggs are graded AA, A and B. AA is the highest just like an A+ is the highest school grade.
The next step in grading is examination of the interior of the egg. This is done by candling or by the breakout method using the Haugh unit system to evaluate the albumen, yolk and air cell.Albumen is judged on the basis of clarity and firmness or thickness. A clear albumen is free from discolorations or from any floating foreign bodies. When an egg is rotated over the candling light, its yolk swings toward the shell. The distinctness of the yolk outline depends on how close to the shell the yolk moves, which is influenced by the thickness of the surrounding albumen. Thick albumen permits limited yolk movement while thin albumen permits greater movement – the less movement, the thicker the white and the higher the grade.
Factors determining yolk quality are distinctness of outline, size and shape and absence of such defects as blemishes or mottling, germ development or blood spots.Higher-grade eggs have shallower air cells. In Grade-AA eggs, the air cell may not exceed 1/8 inch in depth and is about the size of a dime. Grade-A eggs may have air cells over 3/16 inch in depth. There is no limit on air cell size for Grade-B eggs. While air-cell size is considered in grading and eggs take in air as they age, the size of the air cell does not necessarily relate to freshness because size varies from the moment contraction occurs after laying..
Application of protein : Making a protein glue.
A glue can be made from milk by souring it using vinegar (an acid) which separates it into curds and whey. The curds can be neutralised by various bases to produce a variety of different glues. The glue can be tested for strength by sticking together two lolly sticks and attaching weights to them.
Skimmed milk tends to give the best glues. The glue consists of particles of the protein casein that are precipitated from the milk by the adding the acid. It is the polymerisation of these protein molecules that forms the glue. The fat in the milk can get in the way of these polymer chains which is lubricating them like oil does in a bicycle chain and preventing them from sticking together as effectively. Casein is the predominant protein found in fresh milk and cheese. In milk it exists in the form of a soluble calcium salt.
In its acidic form, casein is precipitated by acids such as ethanoic acid (the predominant acid in vinegar). In this experiment, calcium ethanoate is a by-product of the initial souring process, and it is one of the components of the whey solution. The insoluble casein which forms the curds has relatively little secondary or tertiary structure. This means it cannot change its structure. It is relatively hydrophobic, and this is why it is fairly insoluble in water.
In addition to being consumed in milk, casein is used to manufacture adhesives, binders, protective coatings, plastics (such as for knife handles and knitting needles), fabrics, food additives, and many other products. It is commonly used by bodybuilders as a slow-digesting source of amino acids.
GROUP MEMBERS :
FADLIANA NAZIRAH MOHD MUSTAPA D20071029540
NUR AISHAH JAMALUDIN D20071029551
NOOR ASMALINA MAZLAN D20071029564
ASYRAF BIN AHMAD D20071029567
Does the grade of an egg determines the protein content in it?
Labels:
..BiOcHeMisTrY..
- Saturday, August 22, 2009
Subscribe to:
Post Comments (Atom)

0 comments:
Post a Comment